Classification
SM is classified as a myeloid neoplasm by the World Health Organization (WHO).3,a

Systemic Mastocytosis Subtypes3,7
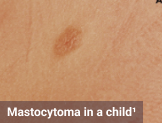
The most common subtype of SM with heterogeneous clinical presentation, which may encompass a range of potentially debilitating symptoms involving the skin, bone, gastrointestinal system, and other organ systems.1-8
SM, systemic mastocytosis.
References:
1. Verstovek S et al. Systemic mastocytosis. In: WHO Classification of Tumours Editorial Board. Haematolymphoid tumours [Internet; beta version ahead of print]. International Agency for Research on Cancer; 2023. (WHO classification of tumours series, 5th ed; vol 11). Accessed September 15, 2023. https://tumourclassification.iarc.who.int/chapters/63 2. Trizuljak J et al. Allergy. 2020;75(8):1923-1934.
3. Sperr WR et al. Lancet Haematol. 2019;6(12):e638-e649. 4. Pardanani A. Am J Hematol. 2023;98(7):
1097-1116. 5. Pyatilova P et al. J Allergy Clin Immunol Pract. 2022;10(8):2015-2024. 6. Lim KH et al. Blood.
2009;113(23):5727-5736. 7. Taylor F et al. Orphanet J Rare Dis. 2021;16(1):414. 8. Jennings SV et al. Immunol Allergy Clin North Am. 2018;38(3):505-525.
hematologic neoplasm
a Please note, these are the anticipated changes to the WHO guidelines, 5th edition, volume 11; cited from the beta version (ahead of print) available online.3
Key SM characteristics

Clonal mast cell proliferation driven by the KIT D816V mutation in
~95% of cases2-6
Abnormal activation of mast cells may lead to potentially
debilitating symptoms, including potentially
life-threatening
anaphylaxis1,8-10
Uncontrolled proliferation and activation of mast cells may result in a wide range of potentially severe symptoms1,8,11,12

Abnormal mast cells8
Release of
pro-inflammatory mediators
(eg, tryptase, histamine, IL-6, TNF)8
Clinical presentation
These signs and symptoms represent the clinical spectrum of systemic mastocytosis and are not all-inclusive. Symptoms may vary in individuals, based on the type of systemic mastocytosis and aggressiveness of the disease.1,3
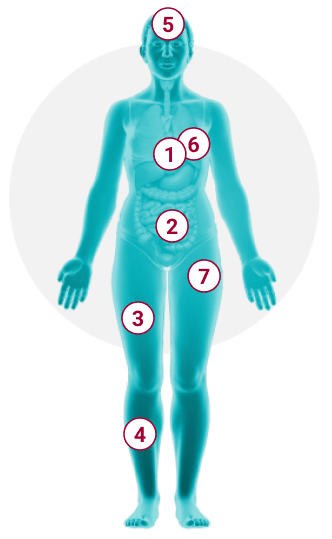
1
Cardiovascular1,3,10
anaphylaxis with
hypotension and syncope, palpitations, angioedema
2
Gastrointestinal1,10,12
nausea, vomiting,
diarrhea, abdominal pain/cramping, heartburn
3
Musculoskeletal1,3
osteopenia, osteoporosis, fractures, back pain, bone
pain,
arthralgias, myalgias
4
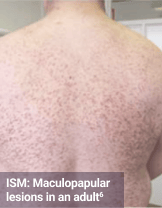
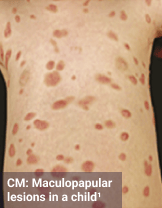
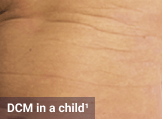
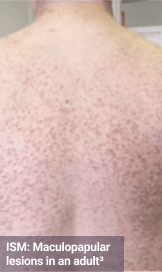
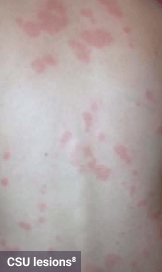
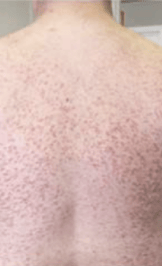
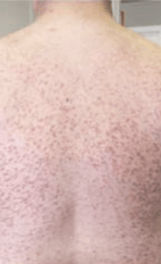
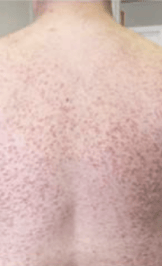
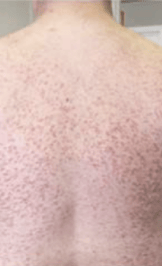
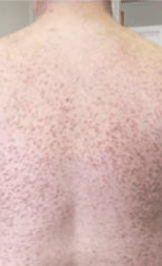
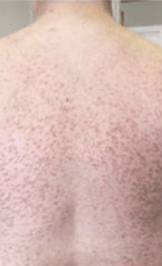
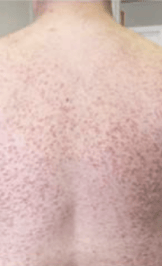
Skin1,3,10,12,13
Darier sign, pruritus, flushing, urticaria, dermatographism
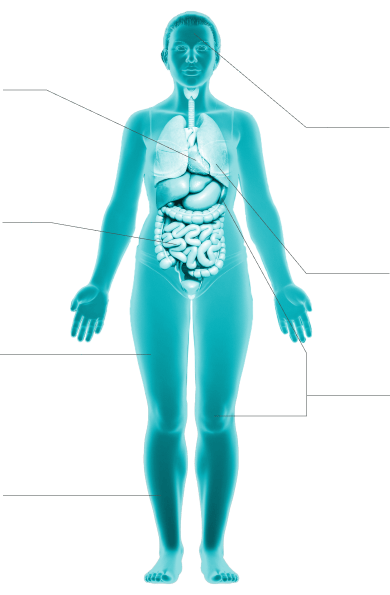
5
Neurocognitive1,10,16-18
memory/cognitive difficulties, brain fog, lack
of focus,
dizziness, depression, headache, sleep disturbance, irritability,
anxiety, migraines
6
Respiratory8,19
wheezing, nasal congestion, throat swelling, dyspnea
7
Systemic1,3,10
anaphylaxis with hypotension and syncope, fatigue,
generalized weakness, sweats, chills, weight loss
Symptoms may result from mast cell mediator release in response to specific triggers, which differ for each patient and may include10,20:
Physical factors16,17,20,21
Heat, cold, changes
in temperature, pressure,
or friction
Odors17,20
Perfumes, chemical exposure
Food or beverages16,17,20,21
Containing or releasing histamine, alcohol
Medications and
therapeutic agents16,17,20,21
Opiates,
NSAIDs, antibiotics, anesthetics, vaccinations,
and contrast media
Insects16,17,20,21
Hymenoptera sting or venom
General triggers16,17,20,21
Environmental allergens, infections, exercise, stress (emotional
or physical),
lack of sleep
NSAIDs, nonsteroidal anti-inflammatory drugs.
ReferencesDiagnosis

WHO Criteria for Systemic Mastocytosis
The WHO diagnostic criteria for SM consist of major and minor criteria.3,a
WHO Systemic Mastocytosis Diagnostic Criteria3,a
(1 major + ≥1 minor or ≥3 minor)
Major criterion
Multifocal dense infiltrates of MCs (≥15 MCs in aggregates) are detected in sections of bone marrow and/or other extracutaneous organ(s)
Minor criteria (anticipated changes)
Activating KIT point mutation(s) at codon
816 or in other critical regions of KITb in bone
marrow or
other extracutaneous organ(s)
Baseline serum tryptase concentration >20 ng/mL in the absence of a myeloid-associated hematologic neoplasm.c In the case of a known HαT, the tryptase level could be adjustedd

Peripheral blood test may be used to screen for SM3,22,23
>25% of all MCs are atypical cells (type I or type II) on bone marrow smears or are spindle-shaped in dense and diffuse MC infiltrates in sections of bone marrow or other extracutaneous organ(s)e
MCs in bone marrow, blood, or another extracutaneous organ(s) aberrantly express 1 or more of the following antigens: CD2, CD25, CD30f
B- and C-findings
Symptoms related to organ infiltration in SM are classified as B (burden of disease) and C (cytoreduction-required) findings reflecting organ dysfunction and disease aggressiveness, which help to define the subtype of SM.3,24
HαT, hereditary alpha tryptasemia; ISM, indolent systemic mastocytosis;
MC, mast cell; SM-AHN, systemic mastocytosis with an associated
hematologic neoplasm; WHO, World Health Organization.
a Please note, these are the anticipated changes to the WHO
guidelines, 5th edition, volume 11; cited from the beta version (ahead
of print) available online.3
b Any type of KIT mutation counts as a minor SM criterion when
solid evidence for its transforming behavior is available.3
c Myeloid neoplasms can lead to increased serum tryptase
levels. Therefore, this criterion does not count in cases of SM-AHN.3
d A possible mode for adjustment has been proposed. The basal
tryptase level may be divided by 1 plus the extra copy numbers of the
alpha tryptase gene.3
e In tissue sections, an abnormal MC morphology counts in both
a dense infiltrate and a diffuse MC infiltrate. In the bone marrow
smear, an atypical morphology of MCs does not count as SM criterion when
MCs are located in, or adjacent to, bone marrow particles. Morphologic
criteria of atypical MCs have been described.3
f Expression has to be confirmed by either flow cytometry or
immunohistochemistry.3
Considerations in the Diagnostic Process
The following diagram is an example of a potential diagnostic workup and is not a specific recommendation or guideline for diagnosing patients with SM.
Recognizing hallmark signs and symptomsa
Dermatologic
Including: Skin lesions,
urticaria, pruritus, flushing1,3,12,13
Systemic
Including: Recurrent or
unexplained anaphylaxis with hypotension and syncope1,3,10
Gastrointestinal
Including: Diarrhea,
bloating, abdominal cramping, nausea, and vomiting1,3,25

Evaluation of SM

Peripheral blood testing for screening of SM
KIT D816V testing with a
high-sensitivity molecular
assay1,3,8,22
Serum tryptase level1,3,8,23
Mast cell expression of 1 or more of the following: CD2, CD25, CD301,3

Biopsy
Bone marrow biopsy or biopsy of an extracutaneous organ1,3,8
SM, systemic mastocytosis.
a Please note, only select signs and symptoms are shown.
Considerations for Systemic Mastocytosis Workup
Click the plus button to reveal answers
MC mediator symptoms should raise suspicion of SM; however, a thorough workup is required for definitive diagnosis.3,10,26
Tryptase >20 ng/mL is considered a minor diagnostic criterion for
SM in the WHO guidelines,a
but basal serum tryptase
levels <20 ng/mL might not rule out SM.1,3,27
BM, blood, or other extracutaneous organ(s) may be screened for
activating KIT point
mutation(s) at codon 816 or in any
other critical regions of KIT. KIT mutation is
considered a key
diagnostic component of SM and is a minor
criterion in the WHO guidelines.1,3,22,a
SM involves proliferation of abnormal MCs, which accumulate in one
or more organ systems,
which may include BM, gastrointestinal
tract, lymph nodes, spleen, liver, or other organs.
Presence of
dense multifocal infiltrates of MCs, defined as ≥15 MCs in
aggregates in sections of
BM and/or other extracutaneous organ(s),
is a major criterion of SM in the WHO guidelines.1,3,24,a
Evaluation of clinical signs of SM, classified as B- or C-findings, can help define the subtype of SM as they indicate organ involvement and aggressiveness of SM, according to the WHO guidelines.3,24,a
B (burden of disease) findings:
0 B-finding? Consider BMM
0-1 B-finding? Consider ISM
≥2 B-findings? Consider SSM
C (cytoreduction-required) findings:
0 C-finding? Consider chronic MCL
0-1 C-finding? Consider ASM
Meets criteria for SM and AHN?
Consider SM-AHN
≥1 C-finding?
Consider acute MCL
AHN, associated hematologic neoplasm; ASM, aggressive systemic
mastocytosis; BM, bone marrow; BMM, bone marrow mastocytosis; ISM,
indolent systemic mastocytosis; MC, mast cell;
MCL, mast cell leukemia;
SM, systemic mastocytosis; SSM, smoldering systemic mastocytosis; WHO,
World Health Organization.
aPlease note, these are the anticipated changes to the WHO
guidelines, 5th edition, volume 11; cited from the beta version (ahead of
print) available online.3
References:
- Pardanani A. Systemic mastocytosis in adults: 2023 update on diagnosis, risk stratification and management. Am J Hematol. 2023;98(7):1097-1116.
- Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7): 1703-1719.
- Verstovek S, Colmenero I, Cozzolino I, et al. Systemic mastocytosis. In: WHO Classification of Tumours Editorial Board. Haematolymphoid tumours [Internet; beta version ahead of print]. International Agency for Research on Cancer; 2023. (WHO classification of tumours series, 5th ed; vol 11). September 28, 2023. https://tumourclassification.iarc.who.int/chapters/63
- Garcia-Montero AC, Jara-Acevedo M, Alvarez-Twose I, et al. KIT D816V-mutated bone marrow mesenchymal stem cells in indolent systemic mastocytosis are associated with disease progression. Blood. 2016;127(6):761-768.
- Kristensen T, Vestergaard H, Bindslev-Jensen C, Møller MB, Broesby-Olsen S. Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am J Hematol. 2014;89(5):493-498.
- Ungerstedt J, Ljung C, Klimkowska M, Gülen T. Clinical outcomes of adults with systemic mastocytosis: a 15-year multidisciplinary experience. Cancers (Basel). 2022;14(16):3942.
- Valent P, Akin C, Gleixner KV, et al. Multidisciplinary challenges in mastocytosis and how to address with personalized medicine approaches. Int J Mol Sci. 2019;20(12):2976.
- Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373(2):163-172.
- Valent P. Risk factors and management of severe life-threatening anaphylaxis in patients with clonal mast cell disorders. Clin Exp Allergy. 2014;44(7):914-920.
- Gilreath JA, Tchertanov L, Deininger MW. Novel approaches to treating advanced systemic mastocytosis. Clin Pharmacol. 2019;11:77-92.
- Rossignol J, Polivka L, Maouche-Chrétien L, Frenzel L, Dubreuil P, Hermine O. Recent advances in the understanding and therapeutic management of mastocytosis. F1000Res. 2019;8:F1000 Faculty Rev-1961.
- Pyatilova P, Akin C, Alvarez-Twose I, et al. Refined treatment response criteria for indolent systemic mastocytosis proposed by the ECNM-AIM Consortium. J Allergy Clin Immunol Pract. 2022;10(8):2015-2024.
- Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma and Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137(1):35-45.
- Aberer E, Sperr WR, Bretterklieber A, et al. Clinical impact of skin lesions in mastocytosis: a multicenter study of the European Competence Network on Mastocytosis. J Invest Dermatol. 2021;141(7):1719-1727.
- Manjaly Thomas ZR, Hartmann K. Skin disease in mastocytosis. In: Akin C, ed. Mastocytosis. Springer, Cham. 2020:69-91.
- Gülen T, Elberink JNGO, Brockow K. Anaphylaxis in mastocytosis. In: Akin C, ed. Mastocytosis. Springer, Cham; 2020:141-155.
- Jennings SV, Slee VM, Zack RM, et al. Patient perceptions in mast cell disorders. Immunol Allergy Clin North Am. 2018;38(3):505-525.
- Scherber RM, Borate U. How we diagnose and treat systemic mastocytosis in adults. Br J Haematol. 2018;180(1):11-23.
- Taylor F, Akin C, Lamoureux RE, et al. Development of symptom-focused outcome measures for advanced and indolent systemic mastocytosis: the AdvSM-SAF and ISM-SAF©. Orphanet J Rare Dis. 2021;16(1):414.
- Jennings SV, Slee VM, Hobart JS, et al. International support and advocacy for mast cell disease patients and caregivers. In: Akin E, ed. Mastocytosis. Springer, Cham; 2020:267-286.
- Castells M, Butterfield J. Mast cell activation syndrome and mastocytosis: initial treatment options and long-term management. J Allergy Clin Immunol Pract. 2019;7(4):1097-1106.
-
Hoermann G, Sotlar K, Jawhar M, et al. Standards of genetic testing
in the diagnosis and prognostication of systemic mastocytosis in 2022:
recommendations of the EU-US Cooperative Group.
J Allergy Clin Immunol Pract. 2022;10(8):1953-1963. - Matito A, Morgado JM, Álvarez-Twose I, et al. Serum tryptase monitoring in indolent systemic mastocytosis: association with disease features and patient outcome. PLoS One. 2013;8(10):e76116.
- Vaes M, Benghiat FS, Hermine O. Targeted treatment options in mastocytosis. Front Med (Lausanne). 2017;4:110.
- Zanelli M, Pizzi M, Sanguedolce F, et al. Gastrointestinal manifestations in systemic mastocytosis: the need of a multidisciplinary approach. Cancers (Basel). 2021;13(13):3316.
- Bibi S, Arock M. KIT and other mutations in mastocytosis. In: Akin E, ed. Mastocytosis. Springer, Cham; 2020:267-286.
- Akin C, Valent P. Diagnostic criteria and classification of mastocytosis in 2014. Immunol Allergy Clin North Am. 2014;34(2):207-218.